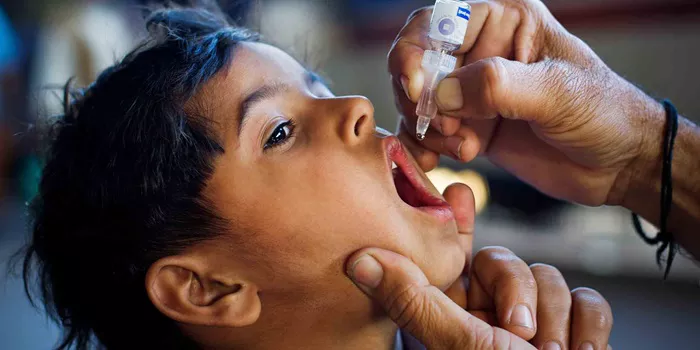
Jerusalem, Amman, Geneva – September 4, 2024 – The initial phase of a critical two-round polio vaccination campaign in central Gaza has concluded successfully, with over 187,000 children under the age of ten receiving the novel oral polio vaccine type 2 (nOPV2). Conducted from September 1 to 3, 2024, this first phase surpassed its target of 157,000 children due to an influx of population into central Gaza and expanded coverage beyond the initial humanitarian pause zone.
To ensure comprehensive coverage, vaccination efforts will continue at four major health facilities in central Gaza over the coming days. Additional vaccine doses have been allocated to these centers to address any unmet needs.
Dr. Richard Peeperkorn, WHO Representative for the occupied Palestinian territory, expressed optimism about the campaign’s progress. “It has been extremely encouraging to see thousands of children receiving polio vaccines, supported by their resilient families and dedicated health workers, despite the challenging conditions over the past 11 months. The respect shown for the humanitarian pause is a positive sign, and we hope this momentum will continue.”
The first phase of the campaign involved 513 teams, comprising over 2,180 health and community outreach workers. Vaccinations were administered at 143 fixed sites, including hospitals, primary care centers, displaced persons camps, public gathering spaces, and along major transit routes. Mobile teams were deployed to reach remote and hard-to-access areas, addressing the needs of families unable to visit fixed sites. Special missions were conducted in areas just outside the humanitarian pause zone, such as Al-Maghazi, Al-Bureij, and Al-Mussader, to ensure coverage for all eligible children.
Preparations are now underway for the second phase of the campaign, scheduled to run from September 5 to 8, 2024, in southern Gaza. This phase aims to reach approximately 340,000 children under ten years old, with 517 teams, including 384 mobile units, set to be deployed. Community outreach workers have already started informing families in southern Gaza about the upcoming vaccination effort. Supplies, including 490 vaccine carriers and 90 cold storage boxes, have been sent to Khan Younis for distribution.
The final phase of the campaign will take place in northern Gaza from September 9 to 11, 2024, targeting around 150,000 children.
To effectively combat the outbreak and prevent further spread, a vaccination coverage rate of at least 90% is required for each campaign phase. Given the severely disrupted health, water, and sanitation systems in the Gaza Strip, continuous monitoring of vaccination coverage will be implemented, with adjustments made as necessary to meet coverage goals.
The two-round campaign is a collaborative effort led by the Palestinian Ministry of Health, in partnership with the World Health Organization (WHO), the United Nations Children’s Fund (UNICEF), the United Nations Relief and Works Agency for Palestine Refugees (UNRWA), and various other stakeholders. The objective is to administer two doses of nOPV2 to approximately 640,000 children in each round.
Dr. Peeperkorn emphasized the importance of continued cooperation, stating, “The successful execution of the first phase in central Gaza is the result of significant coordination among partners, including the Global Polio Eradication Initiative (GPEI) and donors. It highlights the critical role of peace in safeguarding the health and well-being of Gaza’s population. We urge all parties to maintain their commitment to the humanitarian pauses as the second phase begins.”
This campaign is a vital response to a recent outbreak of circulating variant poliovirus type 2 (cVDPV2) in Gaza, which had been polio-free for 25 years. The presence of cVDPV2 was detected in environmental samples collected from central Gaza in June 2024. Four cases of acute flaccid paralysis (AFP) have been reported, including one confirmed case of polio. Two cases tested negative for poliovirus, with laboratory results pending for the fourth case.
The nOPV2 vaccine is specifically designed to combat cVDPV2, the predominant variant in the recent outbreak. It is both safe and effective, providing protection against paralysis and community transmission, and is the recommended vaccine for variant type 2 poliovirus outbreaks globally.
[inline_related_posts title=”You Might Be Interested In” title_align=”left” style=”list” number=”6″ align=”none” ids=”12098,12094,12090″ by=”categories” orderby=”rand” order=”DESC” hide_thumb=”no” thumb_right=”no” views=”no” date=”yes” grid_columns=”2″ post_type=”” tax=””]