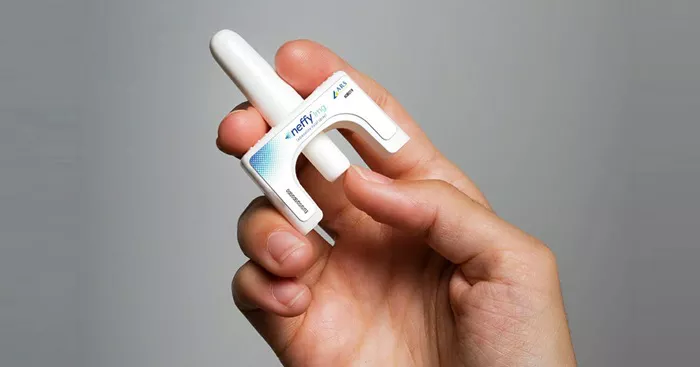
The Food and Drug Administration (FDA) has recently approved Neffy, the first nasal spray designed to deliver epinephrine for emergency treatment of severe allergic reactions, known as anaphylaxis. This approval marks a significant advancement in the management of life-threatening allergies, particularly for children and families who may find traditional injection methods daunting.
Before Neffy’s approval, epinephrine administration during an anaphylactic emergency was only possible via injection, commonly recognized by the brand name EpiPen. However, the injection process can be intimidating, especially for children. The introduction of Neffy is expected to broaden access to epinephrine, providing a more user-friendly option that could alleviate anxiety for many parents and caregivers dealing with severe allergies.
Understanding Anaphylaxis
Anaphylaxis occurs when the immune system reacts abnormally to certain triggers, such as food, medications, or insect stings, leading to a potentially life-threatening reaction. Dr. Katharine Clouser, a pediatric hospitalist at Joseph M. Sanzari Children’s Hospital in New Jersey, explains, “Anaphylaxis is a rapid release of inflammatory cells in response to something the body recognizes as foreign. Unlike a simple allergy that may cause skin reactions or swelling, anaphylaxis affects multiple organ systems.”
The symptoms of anaphylaxis can vary widely. Some individuals experience hives, swelling, itching, or vomiting, while others may have difficulty breathing or even lose consciousness. Dr. Clouser notes that children may not describe symptoms like itchiness in the same way adults do but might exhibit signs such as frequent throat clearing or coughing.
Why Neffy is a Game-Changer
Epinephrine remains the only effective treatment for anaphylaxis, working by relaxing the muscles in the airways. While injectable epinephrine is highly effective, it can be challenging for parents to administer correctly, especially when trying to ensure the needle penetrates deeply enough and is held in place for the required duration.
“The discomfort associated with needles, although lifesaving, can cause additional stress for children,” says Dr. Clouser. “Neffy, as a nasal spray, makes the process of treating anaphylaxis more approachable for families.”
Dr. Clouser also highlights practical challenges associated with injections, such as delivering the medication through thick clothing, which can complicate the process. A nasal spray like Neffy could simplify administration, especially for those who fear needles, potentially reducing treatment delays and increasing the likelihood of timely intervention.
“It might also be easier to administer the nasal spray to a squirming child,” Dr. Clouser adds. “And with a nasal spray, there’s less risk to the person giving the medication since there’s no needle involved.”
Neffy’s Safety and Usage Guidelines
Neffy is approved for use in children weighing over 66 pounds and is administered as a single-dose spray into one nostril. If symptoms do not improve or worsen, a second dose can be administered in the same nostril.
The FDA has listed several common side effects associated with Neffy, including throat irritation, nasal discomfort, headache, and a tingling sensation in the nose. Other potential side effects include dizziness, nausea, and abdominal pain.
However, Neffy is not suitable for everyone. Dr. Clouser advises consulting with a healthcare provider, particularly for children with nasal conditions like polyps or a history of nasal surgery, as these could affect the spray’s absorption. Additionally, individuals with certain coexisting conditions or allergies, such as sulfite sensitivity, should take extra precautions.
“It’s crucial for families to recognize the symptoms of anaphylaxis quickly,” Dr. Clouser emphasizes. “Even if a child has previously tolerated a food, they can still have a severe reaction. And anytime symptoms involve the mouth or throat, families should act swiftly.”
She also underscores the importance of seeking emergency care following the administration of epinephrine, whether through injection or nasal spray, as additional treatment may be necessary.
The introduction of Neffy represents a significant step forward in making life-saving treatment for anaphylaxis more accessible and less intimidating, offering new hope and reassurance for families managing severe allergies.
[inline_related_posts title=”You Might Be Interested In” title_align=”left” style=”list” number=”6″ align=”none” ids=”11339,11335,11249″ by=”categories” orderby=”rand” order=”DESC” hide_thumb=”no” thumb_right=”no” views=”no” date=”yes” grid_columns=”2″ post_type=”” tax=””]