In a groundbreaking study published in the Proceedings of the National Academy of Sciences, scientists have utilized an innovative approach to uncover the intricate process of protein folding. By translating molecular data into auditory signals, researchers shed light on the rapid transformations that occur when a string of amino acids transitions from an unfolded to a folded state.
Led by University of Illinois Urbana-Champaign chemistry professor Martin Gruebele, in collaboration with composer and software developer Carla Scaletti, the study delves into the critical role of hydrogen bonds in facilitating the lightning-fast gyrations that enable protein folding. Gruebele emphasizes the importance of proper protein folding for its functionality in various biological processes, highlighting the implications of misfolded proteins in diseases like Alzheimer’s, Parkinson’s, and cystic fibrosis.
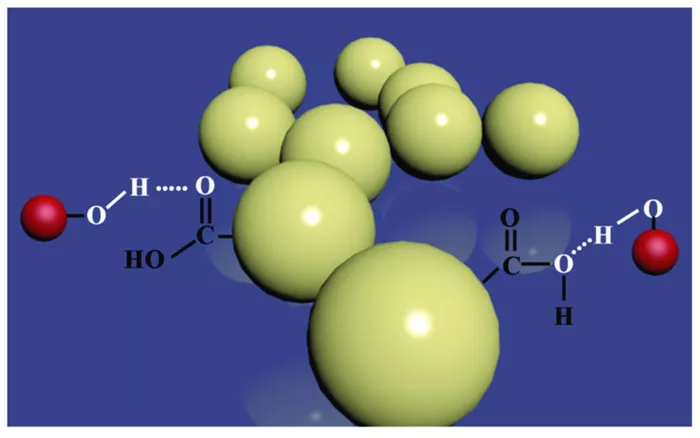
Hydrogen bonds, weak attractions aligning atoms across different amino acids, play a pivotal role in the folding process. However, visualizing these complex events proved challenging for researchers. To overcome this hurdle, the team turned to data sonification, a method converting molecular data into sound. Scaletti developed a software program assigning each hydrogen bond a unique pitch, allowing researchers to “hear” the bonds forming.
The auditory representation of hydrogen bonds provided crucial insights into the folding process, revealing how certain bonds accelerate folding while others impede it. By characterizing these transitions as “highway,” “meander,” and “ambiguous,” researchers gained a deeper understanding of protein folding dynamics.
Incorporating water molecules into the simulations was vital for comprehending the process, as Gruebele explains that a significant portion of energy in protein folding reactions originates from water. While hydrogen bonds are not the sole determinant of protein folding, they often stabilize transitions between folded states, with some bonds temporarily hindering proper folding.
The study, supported by the National Science Foundation, National Institutes of Health, and Symbolic Sound Corporation, underscores the significance of auditory analysis in unraveling the complexities of protein folding. Gruebele emphasizes the auditory patterns revealed through sonification, offering a novel perspective that complements traditional visualization methods.
Gruebele, also affiliated with the Beckman Institute for Advanced Science and Technology and the Carl R. Woese Institute for Genomic Biology at the University of Illinois Urbana-Champaign, anticipates that this auditory approach will enhance understanding of protein folding mechanisms and aid in developing targeted therapeutic interventions for protein folding-related diseases.